
Alteogen, the top bio company by market capitalisation on the KOSDAQ, revealed on the 26th that the counterparty for the option contract for the ALT-B4 technology transfer (LO; License Out) is likely to be the Australian big pharma CSL.
There is also an interpretation that the counterparty has entered into a priority maintenance option contract due to concerns that the LO (License Out) contract order may be delayed. This indicates that big pharma companies are lining up to acquire Alteogen's technology.
Shinhan Investment Corp commented on the 28th regarding Alteogen's option contract signing and stated this.
Shinhan Investment Corp stated, "Alteogen's ALT-B4 has already signed more than 10 material transfer agreements (MTA)" and added, "If the pending deals are signed with a gap of about 5-6 months, the 10th company will require a waiting time of 4 years. Naturally, no company will wait for 4 years."
They continued, "While contracts are being discussed in order, Alteogen typically requires a termsheet within 6 months after signing an MTA" and speculated, "The company that signed this option contract has agreed to pay the option contract amount to maintain the priority in the situation where the LO order has passed this 6-month period."
Ultimately, they concluded that "global pharmaceutical companies are paying and lining up."
They added, "Alteogen has not disclosed the product with the partner with whom it signed the option contract, but it describes it as a modality other than single antibodies, dual antibodies, and ADC, which has a sales potential of billions of dollars annually" and predicted, "Considering the hint that there are competing products in other modalities, the Australian CSL's immunoglobulin IV (intravenous) formulation Privigen is the top priority."
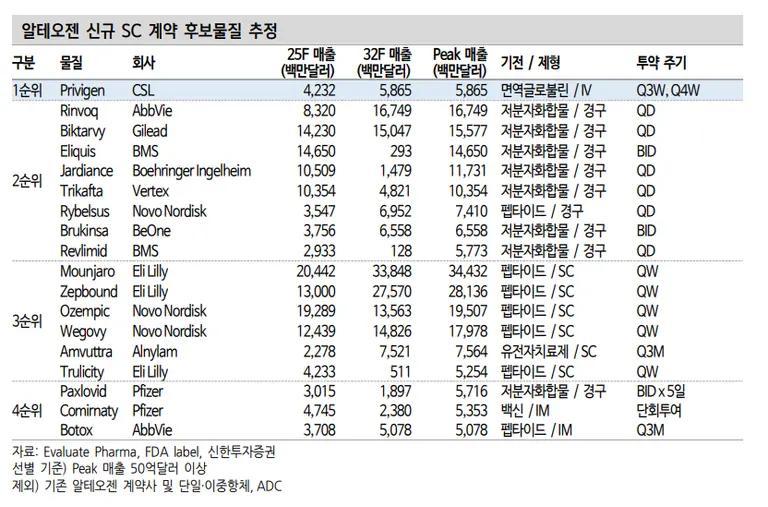
CSL, headquartered in Melbourne, is estimated to generate about 22.5 trillion won in sales this year. Privigen is a key item expected to generate 6 trillion won in sales. Meanwhile, it is in a position of being chased by Takeda's HYQVIA, which has adopted Halozyme's SC conversion technology.
Shinhan Investment Corp stated, "The FDA approval of Keytruda SC and AstraZeneca's LO has delayed the additional LO contracts for ALT-B4" and added, "This is because the first contract conditions after commercialisation will be applied as basic conditions for contracts with more than 10 companies that are in the process of MTA, which makes the first contract conditions important and has caused some delays in the signing process due to advantageous negotiations."
However, they expressed optimism that "after the first LO, contracts can be made under similar conditions with other companies, so the contract term will be significantly shortened."
They continued, "The goal of signing 2 contracts this year has been somewhat delayed, and the stock price is in a correction phase due to patent issues in Germany" and added, "However, patent litigation is a natural process for commercialisation companies, and if existing contract partners do not return the materials, there will be no patent issues, and subsequent continuous LO signings will alleviate concerns."





댓글 (0)
Post Comment